Introduction
Welcome to my comprehensive exploration of the aluminum oxide formula. In this guide, I’ll walk you through some of the basics of aluminum oxide, or Al₂O₃, often highlighted in discussions about ceramics, abrasives, and advanced machining applications. I decided to put this piece together after working with various industrial materials and noticing how frequently people mention aluminum oxide but rarely dive into the real nitty-gritty of its composition, properties, and practical uses.
I’ve spent time in a small workshop where I experimented with different types of abrasives, including Al₂O₃-based sandpapers and grinding wheels. Along the way, I found that many folks—myself included at one point—don’t always understand the significance of the aluminum oxide formula in everyday scenarios. While it can appear technical, this topic is genuinely accessible once you see how it shapes so many aspects of manufacturing, from tool fabrication to protective coatings. In the course of my experimentation, I’ve also realized how valuable Custom Machining techniques can be for maximizing the performance benefits of aluminum oxide, especially when it comes to improving the durability of CNC machined parts. By combining Al₂O₃-enhanced abrasives with precise CNC processes, it’s possible to achieve superior surface finishes that stand up to intense wear and tear.
Before we dig into the extended chapters, let me give you a quick preview of what’s to come. We’ll talk about how the aluminum oxide formula (Al₂O₃) is structured, why it’s so stable, and how it influences different machining processes. We’ll also explore real-world applications, compare notes on best practices, and reflect on how I personally discovered the value of aluminum oxide formula in my own hands-on experiences.
In short, if you’re curious about “aluminum oxide formula” and want a deep dive that balances scientific explanation with practical, workshop-level insights, you’re in the right place. This article is meant for anyone—machinists, DIY enthusiasts, material science students, or just the curious among us who keep seeing “Al₂O₃” in product specifications but aren’t sure why it’s such a big deal.
So, let’s begin. Below, I’ll break down everything into clear chapters. My hope is that by the end, you’ll have a detailed understanding that makes you comfortable discussing aluminum oxide formula in any setting, whether at a professional conference or among friends at a local makerspace.
Chapter 1: Understanding the Core of Al₂O₃
1.1 Quick Overview of Aluminum Oxide Formula
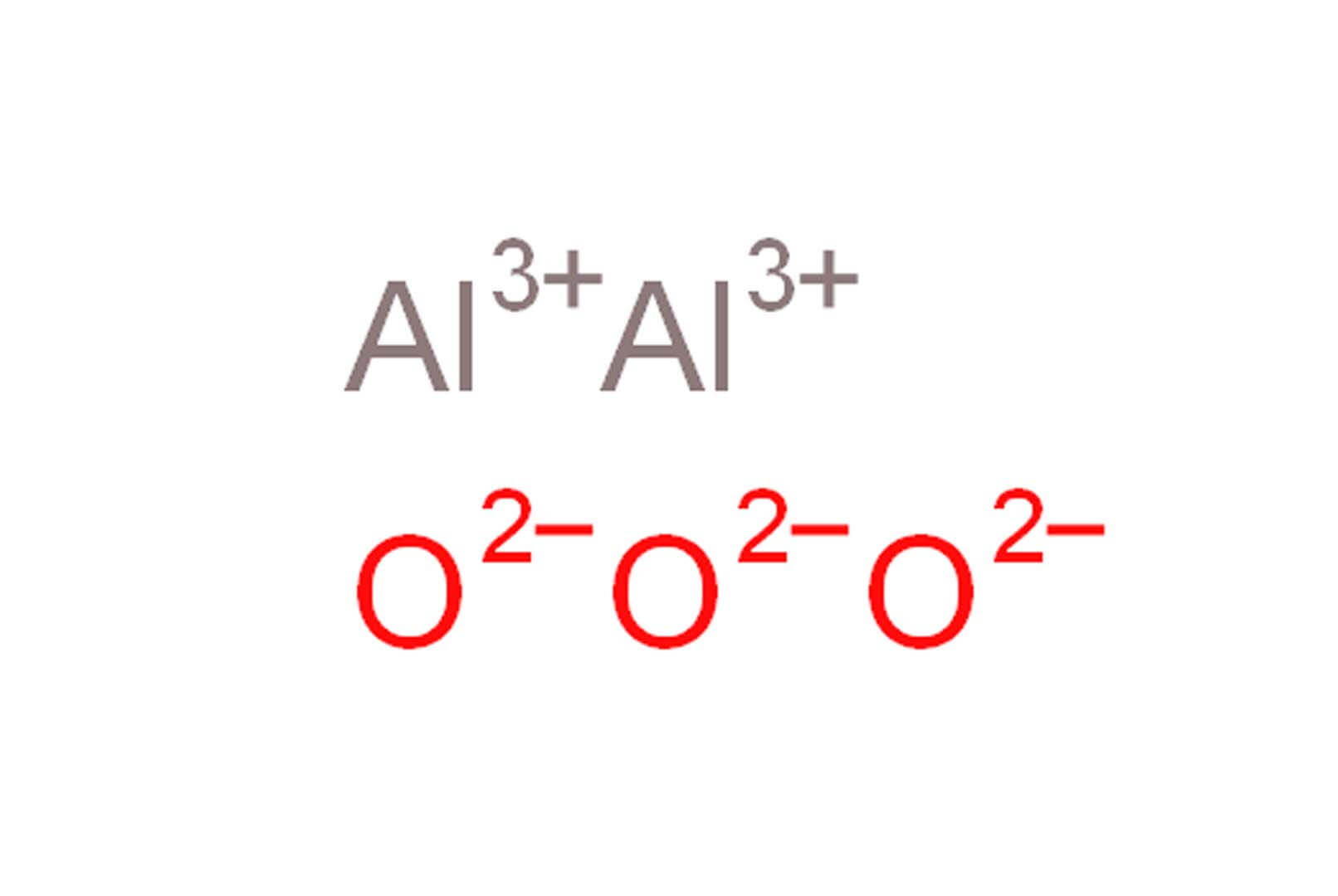
The aluminum oxide formula is Al₂O₃. In words, it’s made up of two aluminum atoms and three oxygen atoms. While it’s easy to memorize, I’ve learned that the real magic is in the way these atoms bond. The aluminum cations (Al³⁺) and oxygen anions (O²⁻) create a lattice that is strong, stable, and resistant to many chemical reactions.
I still remember the first time I properly looked at an Al₂O₃ crystal under a microscope in a materials science lab. The crystalline structure was impressive, with high symmetry. This was not just a random arrangement of atoms; it was a precise, geometric network that gives aluminum oxide its characteristic hardness.
1.2 The Significance of Al₂O₃ in Everyday Life
Aluminum oxide might sound niche, but it’s actually everywhere. You’ll find it in ceramic coatings, abrasives like sandpaper, spark plug insulators, cutting tools, and sometimes even in cosmetics and medical implants. Whenever you see “ceramic” in a high-performance context—especially for cutting or abrasion—there’s a good chance it’s Al₂O₃ or a related compound.
In my case, I often see aluminum oxide formula references when I’m selecting the right abrasive for finishing wood or metal parts. The way Al₂O₃ breaks down into new sharp edges under friction makes it an ideal grain for grinding and sanding applications.
1.3 The Role of Aluminum Oxide Formula in Corrosion Resistance
One of the best-known facts about aluminum is that it doesn’t rust like iron. This is largely because it forms a thin, protective coating of aluminum oxide on its surface. The tight aluminum oxide formula—Al₂O₃—acts as a barrier, preventing oxygen from penetrating deeper and causing further corrosion or oxidation.
When I started tinkering with different metals for a home project, I learned the value of aluminum’s protective oxide layer pretty quickly. It was amazing to see how a well-formed oxide layer could keep a piece of metal in good condition, even in outdoor conditions. If that surface is scratched or removed, the aluminum can oxidize again, creating a new protective layer.
1.4 Why This Guide Matters
The main reason I’m writing this is to clarify all the intricacies behind the aluminum oxide formula. People often ask, “What is the aluminum oxide formula?” or “Why is Al₂O₃ so prevalent in manufacturing?” but rarely get a deep enough answer that addresses both the science and the practical side.
I believe that once you understand the fundamental properties of Al₂O₃, you’ll be better equipped to choose the right materials, interpret engineering specs, or even craft more effective solutions for your own projects.
Chapter 2: The Fundamentals of the Aluminum Oxide Formula
2.1 Introduction to Crystalline Structures
To grasp the fundamentals of aluminum oxide formula, we need to understand crystalline structures. Generally, ionic compounds like Al₂O₃ form structured lattices. Because Al³⁺ (aluminum ions) and O²⁻ (oxide ions) differ in charges, they arrange themselves in a tightly packed, ordered pattern. Each ion is surrounded by oppositely charged ions in such a way that it maximizes electrostatic attraction while minimizing repulsion.
When I first encountered a sample of high-purity Al₂O₃ in a university lab, I was struck by how uniform it looked under an electron microscope. That uniformity is key to its strength and stability, which ties directly into the fact that it’s frequently used as an abrasive and as a protective ceramic.
2.2 Ionic Bonds and Their Strength in Al₂O₃
Aluminum oxide formula, Al₂O₃, points to a ratio where two aluminum ions are paired with three oxide ions. Aluminum typically has a +3 oxidation state, while oxygen is typically -2. The ionic bonds in Al₂O₃ are exceptionally strong because the difference in electronegativity between aluminum and oxygen is quite significant.
You can compare this to something like NaCl (table salt), where the difference in charge is smaller and the overall structure is not as robust under high temperature or extreme mechanical stress. In the case of Al₂O₃, the robust ionic bonds make the crystal resistant to high-temperature environments and mechanical wear.
From a personal standpoint, I remember trying to sand down a hardened piece of steel with a cheap abrasive. It didn’t work well. When I switched to an aluminum oxide abrasive, I could feel the difference in how quickly and efficiently it removed material. That’s partially a reflection of the strong ionic bonds and hardness that come with Al₂O₃.
2.3 Different Phases of Al₂O₃
It might surprise some people to learn that aluminum oxide exists in multiple phases or polymorphic forms. The primary ones are alpha (α), gamma (γ), delta (δ), and there are others such as θ (theta) and κ (kappa). However, the alpha phase (α-Al₂O₃) is the most stable and the most common in industrial applications.
- Alpha (α): This is the stable form of aluminum oxide at high temperatures. It’s extremely hard and is often used for abrasives and cutting tools.
- Gamma (γ): Has a more porous structure, often used in catalytic applications and as a support material in chemical processes.
- Delta (δ) & Theta (θ): Less common phases that tend to appear as transition phases when γ-Al₂O₃ converts to α-Al₂O₃ under heat.
I had the opportunity to see a furnace run in a ceramics class where we gradually heated a sample of γ-Al₂O₃. By the time we reached certain high temperatures, we ended up with mostly α-Al₂O₃. Monitoring this transformation showed me how the exact same “aluminum oxide formula” can behave differently depending on its crystal structure.
2.4 Physical and Mechanical Properties
Aluminum oxide’s mechanical properties are largely the result of its crystal structure and ionic bonding. Here’s a simple table outlining typical properties of α-Al₂O₃. This table should help anchor our discussion:
| Property | Typical Value | Notes |
|---|---|---|
| Density | ~3.95–4.1 g/cm³ | Denser than many ceramics |
| Mohs Hardness | 9 | Next only to diamond (10) and a few other superhard materials |
| Melting Point | ~2,072 °C | Extremely high, useful in high-temperature applications |
| Thermal Conductivity | ~30 W/m·K (varies by purity) | Moderate conductivity for a ceramic |
| Electrical Resistivity | High (insulator) | Useful as an electrical insulator in many devices |
| Compressive Strength | Very high (2,000–4,000 MPa) | Excellent for load-bearing ceramics |
| Fracture Toughness | Moderate, ~3–4 MPa·m½ | Not as high as metals, but good for a ceramic |
| Phase Stability | α-phase stable at high temp | Gamma phase transitions to alpha above ~1,100–1,200 °C |
(Table 2.1: Key properties of α-Al₂O₃. These values can vary based on purity and specific manufacturing processes.)
As you can see, the aluminum oxide formula yields a substance with remarkable hardness, high melting point, and excellent wear resistance. This explains its popularity in industrial sectors that need materials to withstand friction, impact, or extreme heat.
2.5 Importance in Protective Coatings
Because of its corrosion resistance and hardness, Al₂O₃ is used extensively as a protective coating. I recall a project where we used a thermal spray gun to deposit thin layers of aluminum oxide onto metal parts that would face abrasive environments. The result was a significant improvement in lifespan. This is a prime example of how “aluminum oxide formula” translates into real-world durability.
2.6 How Purity Affects Performance
Purity is a big deal in aluminum oxide. Impurities can alter melting points, color, hardness, and even electrical properties. For example, sapphire is essentially pure Al₂O₃ with trace impurities of other elements that create different colors. In an industrial context, even small levels of impurities can affect hardness or brittleness.
When I was looking for an abrasive wheel for my own workshop, I found that wheels labeled as “white aluminum oxide” typically had a higher purity and finer grit than standard brown or pink variants. This wasn’t just marketing fluff—testing them side by side, I noticed that white aluminum oxide abrasives lasted longer and produced a more refined surface finish on hardened steel. This underscores how the aluminum oxide formula’s performance is closely linked to purity.
2.7 Common Industries Relying on Aluminum Oxide
While it’s not an exaggeration to say that nearly every industry touches aluminum oxide formula in one way or another, a few sectors are especially reliant:
- Abrasives and Grinding: The hardness and durability of Al₂O₃ make it ideal for grinding wheels, sandpaper, and other polishing tools.
- Ceramic Parts: Engineered ceramics in aerospace, automotive, and electronics often use Al₂O₃ as the base material.
- Electronics: Because of its excellent electrical insulation, Al₂O₃ is used in circuit boards, substrates, and resistor cores.
- Medical: Inertness and biocompatibility make it suitable for implants and prosthetic devices.
- Chemical Processing: As a catalyst or a catalyst support, especially in the γ-phase form.
When I visited a manufacturing plant that specialized in advanced ceramic parts, I was surprised at how many processes started with a fine aluminum oxide powder. They formed it into intricate shapes, sintered at high temperatures, and ended up with components that could handle extreme conditions in engines and industrial pipelines.
2.8 Environmental Aspects
From an environmental standpoint, aluminum oxide is considered relatively stable and non-toxic. It doesn’t readily leach harmful chemicals into the environment under normal conditions. Of course, mining and refining aluminum does have an environmental impact, but Al₂O₃ itself, once it’s part of a product, doesn’t pose the same risks as some other compounds might.
I once attended a conference on sustainable manufacturing where participants discussed the potential for recycling and reusing ceramic materials, including Al₂O₃-based components. While reclaiming and recycling advanced ceramics can be challenging, the inert nature of Al₂O₃ often means it can be repurposed or safely disposed of in controlled conditions.
2.9 A Peek into Market Dynamics
Demand for aluminum oxide continues to grow across industries. Abrasives alone account for a huge chunk, with electronics and medical applications following close behind. The cost of high-purity Al₂O₃ can be quite substantial, reflecting the complexity involved in producing it at scale without contaminants.
Based on reports I read, global consumption of industrial ceramics (with aluminum oxide at the forefront) has been steadily increasing. This trend aligns with the push for more durable, heat-resistant, and corrosion-resistant materials.
2.10 Summary of Chapter 2
The fundamentals of aluminum oxide formula hinge on its strong ionic bonding, stable crystal structure, and wide range of properties that make it essential across multiple industries. Whether it’s the alpha phase’s incredible hardness or the gamma phase’s usefulness in chemical catalysis, Al₂O₃ is versatile and reliable.
In practical terms, it’s the reason you can buy abrasives that cut through metal efficiently, and it’s the reason aluminum can maintain its corrosion resistance in moist environments. For me, understanding these aspects at a deeper level has transformed how I pick supplies for my own workshop projects. In the next chapter, we’ll dig deeper into the composition, processing, and the ways in which raw aluminum oxide is turned into consumable or industrial-grade products.
Chapter 3: From Powder to Product — Composition & Processing
3.1 Starting with Bauxite
When we talk about “aluminum oxide formula” in an industrial sense, we usually trace it back to bauxite. Bauxite is the primary ore of aluminum. It typically contains varying amounts of hydrated aluminum oxides, iron oxides, and other impurities. The first crucial step in turning bauxite into usable aluminum oxide is refining.
I once toured an alumina refinery that took raw bauxite and subjected it to the Bayer process. Essentially, the bauxite is dissolved in sodium hydroxide, which separates the aluminum-bearing minerals from other components. The result is alumina (Al₂O₃·xH₂O) in a more refined form. After calcination, you get near-pure aluminum oxide.
3.2 Calcination and Phase Transformations
Calcination involves heating the hydrated alumina to temperatures exceeding 1,000 °C. During this heating, water molecules are driven off, and the structure begins transforming. Gamma alumina may form first, with alpha alumina appearing at even higher temperatures (roughly 1,100–1,200 °C). The final product’s properties will depend significantly on how thoroughly this process is done and what impurities remain.
I recall speaking with an engineer about how controlling the calcination temperature can tweak the pore structure of the final Al₂O₃. That’s critical for applications like catalysts, where surface area matters enormously. For abrasives, though, high-density alpha alumina is often preferred due to its hardness and toughness.
3.3 Purity Levels and Their Impact
After calcination, producers can achieve different purity levels. Some processes produce standard “brown” aluminum oxide, which contains roughly 95% Al₂O₃ along with iron oxides and other trace elements. Others yield “white” aluminum oxide, which can exceed 99% Al₂O₃ purity.
Below is a data table showing typical purity levels for various commercial grades of Al₂O₃:
| Grade Name | Typical Al₂O₃ Content | Other Notable Impurities | Common Uses | Approx. Cost (Relative) |
|---|---|---|---|---|
| Brown Al₂O₃ | ~94–96% | Iron oxide, silica | General-purpose abrasives, blasting | Low |
| White Al₂O₃ | 99%+ | Minimal iron, some Na₂O | Precision abrasives, finer finishing | Moderate |
| Pink Al₂O₃ | ~96–97% | Chromium oxide, others | Specialized grinding wheels | Moderate |
| Zirconia-Alumina | ~85% Al₂O₃ + ZrO₂ | Additives to improve toughness | High-end grinding, special cutting | High |
| Tabular Alumina | 99.5%+ | Very low impurity levels | Refractories, high-performance uses | High |
| Fused Alumina | 97–99% | Depending on feed material | Sandblasting, surface preparation | Moderate |
| Reactive Alumina | ~99% | Specifically designed for ceramics | Advanced refractories, fine ceramics | High |
(Table 3.1: Common commercial grades of aluminum oxide and their typical applications.)
When I was sourcing abrasives for a project that required ultra-fine finishing on stainless steel, I opted for a higher-purity white aluminum oxide. The difference in the final surface quality was substantial. It also introduced less contamination, which was especially important because stainless steels are easily discolored by iron contamination.
3.4 Shaping and Forming Techniques
Once you have your desired grade of Al₂O₃ powder, the next step is turning it into a usable product. For ceramics, this often involves pressing, extrusion, or injection molding of Al₂O₃ powder mixed with binders. The shapes are then sintered—heated to near melting point—to fuse the particles into a solid mass.
During sintering, the pores between particles shrink, leading to a dense, solid structure. The sintering temperature can be anywhere from 1,500 °C to over 1,700 °C, depending on the exact formulation.
I recall a visit to a workshop where we produced custom ceramic parts by mixing Al₂O₃ with a resin binder, pressing it into molds, and then firing in a high-temperature kiln. It was fascinating to see how the same “aluminum oxide formula” that I’d encountered as an abrasive could also become a structural ceramic part for high-stress applications like mechanical seals.
3.5 Fused vs. Sintered Aluminum Oxide
In some cases, Al₂O₃ is fused by melting it in an electric arc furnace at temperatures around 2,000 °C. The molten alumina is then cooled and crushed to form abrasive grains. Fused alumina is popular in grinding wheels and blasting media due to its toughness and blocky grain shape.
Sintered alumina, on the other hand, is often more uniform in microstructure. For cutting tools, sintered ceramics with additives like zirconia can significantly improve fracture toughness. When I was selecting an ultra-durable cutting insert, I came across sintered Al₂O₃-based ceramics that were specifically engineered to machine exotic alloys at high speeds.
3.6 Coatings and Thin Films
Aluminum oxide doesn’t just exist as bulk ceramics or abrasives; it’s also used as a thin coating on metal substrates. Techniques like plasma spraying, chemical vapor deposition (CVD), and physical vapor deposition (PVD) can deposit Al₂O₃ layers only a few micrometers thick. This approach is common in cutting tools, where an Al₂O₃ coating can extend tool life by reducing heat transfer and friction.
Early in my career, I was intrigued by the dramatic improvement in tool life once a CVD Al₂O₃ coating was applied. The tool edges suffered less wear, and we could run at higher cutting speeds without the tool’s edge degrading quickly. The coating’s thickness was often less than 20 microns, yet it had an enormous impact.
3.7 Role of Additives and Dopants
To modify properties for specific applications, manufacturers dope Al₂O₃ with small amounts of other oxides like MgO, Y₂O₃, or TiO₂. These additives can improve sinterability, grain growth control, or even color. For instance, doping with magnesium oxide can inhibit grain growth in sintering, resulting in finer-grained ceramics that are stronger and more reliable.
I once tested two batches of Al₂O₃ parts—one doped with a small amount of magnesium oxide and one without. The doped batch exhibited a significantly more consistent microstructure under microscopy, and it performed better in mechanical tests. This taught me how sensitive the performance of the aluminum oxide formula can be to even small compositional changes.
3.8 Processing Defects and Quality Control
Despite the robustness of Al₂O₃, processing defects can arise. Common issues include:
- Porosity: Incomplete sintering can leave pores that weaken the part.
- Grain Boundary Impurities: Contaminants can concentrate at grain boundaries, leading to decreased strength.
- Thermal Shock Cracks: Rapid heating or cooling can crack ceramics with poor thermal shock resistance.
Quality control methods include ultrasonic inspections, microstructural analysis, and even acoustic resonance testing. I watched a demonstration where a small ceramic rod was subjected to a “ring test.” By tapping it and listening to the resonance, skilled operators could detect subtle defects.
3.9 Economic Considerations in Production
Producing high-purity Al₂O₃ can be expensive due to the energy-intensive processes involved (e.g., high-temperature sintering or fusion). Costs vary widely based on purity, particle size, shape, and any additional processing steps like doping or coating.
From a business perspective, balancing cost with performance is always a challenge. For example, a foundry might prefer a lower-cost brown alumina for general blasting, but a tool manufacturer making specialized high-speed inserts may require top-grade sintered alumina. When I was helping a small machine shop select abrasive media, they had to weigh the higher cost of white aluminum oxide against the improved surface finish and lower machine downtime that came from fewer part reworks.
3.10 Personal Insights on Working with Al₂O₃
Over the years, I’ve had the chance to handle aluminum oxide in various forms. Here are some personal takeaways:
- Dust and Safety: Fine Al₂O₃ powder can be an inhalation hazard. Proper masks and ventilation are essential in processing environments.
- Machinability: Fully sintered Al₂O₃ ceramics are extremely hard to machine. We typically shape them in the green or semi-fired state, or use diamond tools if we need to do post-sintering finishing.
- Customization: Modern industry can tailor Al₂O₃’s properties through doping, grain size control, and composite approaches. This means the same “aluminum oxide formula” can lead to drastically different end products.
3.11 Chapter 3 Summary
This chapter delved into the route from raw ore (bauxite) to refined powders, and from powders to finished Al₂O₃ products. We covered key distinctions like fused vs. sintered alumina, the importance of purity, and the role of doping.
Ultimately, understanding how aluminum oxide is processed brings us closer to appreciating its adaptability. The same material can appear as a tough abrasive, a protective coating, or a highly specialized ceramic component, all thanks to variations in manufacturing techniques and purity levels. It’s this adaptability that cements Al₂O₃’s status as a cornerstone in modern industry.
Chapter 4: Machining with Al₂O₃ — Tools, Techniques, and Tips
4.1 Introduction to Machining Ceramics
Machining ceramics has always posed a unique challenge because of their brittleness and high hardness. Traditional metal-cutting methods don’t always translate well when it comes to ceramics. Yet aluminum oxide formula (Al₂O₃) has carved out a robust niche in cutting and finishing tools.
My early experiences involved using a standard steel cutting tool on a small block of sintered Al₂O₃. It dulled the steel edge almost immediately. That’s when I learned that you often need diamond or cubic boron nitride (CBN) tools for direct ceramic machining.
4.2 Al₂O₃ as a Cutting Tool Material
Aluminum oxide can also be the cutting tool itself. A typical “ceramic insert” for lathes or milling machines may be composed primarily of Al₂O₃. These ceramic inserts can withstand higher cutting speeds on certain materials, such as cast iron or hardened steels, due to their superior hot hardness.
When I tested an Al₂O₃-based ceramic insert on a lathe for a finishing pass on hardened steel, the longevity was impressive compared to carbide inserts. The chip formation was consistent, and I was able to increase cutting speed by 30% without quickly wearing out the tool. That’s part of the reason why “aluminum oxide formula” has become synonymous with advanced machining.
4.3 Tool Geometry and Setup
Ceramic tools, including those made from Al₂O₃, often require specific geometries to minimize the forces that cause fracturing. Sharp edges can be detrimental in some cases because they concentrate stress. Instead, a small chamfer or T-land may be applied to the cutting edge to distribute cutting forces more evenly.
In my workshop, I had to adjust the tool holder angles when I first experimented with ceramic inserts. The geometry that worked for carbide wasn’t ideal for Al₂O₃. I consulted reference materials from the insert manufacturer and found that a slightly negative rake angle helped stabilize the cutting forces.
4.4 Cooling and Lubrication Concerns
Ceramic inserts are known for their ability to handle high temperatures. In some cases, it’s even recommended to machine dry (with no coolant) to avoid thermal shocks. Rapid temperature changes can crack the ceramic. However, if coolant is used, it must be steady and properly directed to keep the tool at a uniform temperature.
I recall once spraying coolant intermittently on an Al₂O₃ insert. It led to premature cracking. After that incident, I realized how sensitive ceramics can be to sudden temperature differences.
4.5 Grinding and Polishing with Al₂O₃ Abrasives
Aluminum oxide formula is the backbone of countless grinding wheels, sanding discs, and polishing media. These abrasives come in various grit sizes, bond types, and hardness levels:
- Vitrified-bond wheels: Common for precision grinding.
- Resin-bond wheels: Often used for finish or fine grinding.
- Coated abrasives (sandpaper): For a wide range of applications from woodworking to metal finishing.
I’ve personally used aluminum oxide sandpaper to polish everything from soft woods to hardened steels. The key is selecting the correct grit. Coarser grits remove material quickly but can scratch the surface, whereas finer grits provide a smoother finish.
4.6 Optimal Machining Parameters
Choosing the correct machining parameters (speed, feed, depth of cut) is critical when using Al₂O₃-based tools or abrasives. Speeds are generally higher than for carbide tools, but feeds and depths are often lower to reduce the risk of sudden fracture.
In one machining trial, I gradually increased cutting speed on a hardened steel part. The ceramic insert produced fine, almost powdery chips at high speed. This contrasted with carbide inserts that typically formed heavier, curled chips. The phenomenon occurs because ceramics can maintain hardness at temperatures that would soften carbide tools.
4.7 Safety and Best Practices
- Dust Collection: Grinding and polishing with Al₂O₃ abrasives can create fine dust. A proper dust collection system or mask is vital.
- Handling Ceramics: Ceramic inserts can chip or crack if dropped. I learned the hard way, breaking a brand-new insert by accidentally knocking it off the bench.
- Thermal Management: As mentioned, consistent cooling (or intentionally no cooling) is important to prevent thermal shock.
4.8 Case Study: High-Speed Machining of Cast Iron
A friend of mine runs a small machine shop focusing on automotive components. He switched from carbide inserts to Al₂O₃ ceramic inserts for finishing cast iron brake rotors. After some experimentation, he found:
- A 20–30% reduction in cycle time due to higher cutting speeds.
- Longer tool life, even though each insert cost more.
- Fewer tool changes, reducing downtime.
This shift not only saved money in the long run but also improved product consistency. It’s a textbook example of how leveraging the aluminum oxide formula can yield tangible industrial benefits.
4.9 Common Pitfalls in Machining with Al₂O₃
- Using Standard Holders: Ceramic inserts often need specialized tool holders. Failing to provide adequate support can lead to chipping.
- Excessive Feed Rates: Ceramics can handle speed, but not always heavy feeds. Overloading can cause catastrophic failure.
- Shock Loads: Interrupted cuts or heavily scaled surfaces can shock the tool. I usually recommend smoothing or cleaning the workpiece if it’s heavily scaled before using a ceramic insert.
4.10 My Personal Reflection on Al₂O₃ Machining
I initially found ceramic tooling intimidating because of its brittleness. But once I got the knack for setting the right speeds, feeds, and tool paths, I realized the potential was enormous. It felt almost magical to watch the tool cut through hardened steel with minimal wear.
That said, it’s not for every situation. For heavy roughing or interrupted cuts, carbide or cermet tools might still be a better bet, at least in my experience. But whenever high-speed finishing of cast iron or hardened steel is on the table, Al₂O₃ stands out.
4.11 Chapter 4 Summary
Machining with Al₂O₃, whether as a tool material or an abrasive, offers unique advantages: high-speed capability, excellent wear resistance, and fine surface finishes. The aluminum oxide formula underpins this performance, thanks to its robust ionic bonds and thermal stability.
However, success requires understanding the specific geometry, cooling strategy, and feed/speed parameters that suit ceramics. Done right, it can dramatically improve productivity. Done wrong, it can end in cracked tools and frustration.
The next chapter will expand on how aluminum oxide products fit into a broader range of real-world applications, spanning everything from aerospace parts to household items.
Chapter 5: Real-World Applications and Industry Insights
5.1 Aerospace Components
Aluminum oxide formula is front and center in many high-performance aerospace applications. Whether it’s for engine components, thermal protection systems, or even the manufacturing of cutting tools that shape lightweight aluminum alloys, Al₂O₃ is a frequent flyer. Its ability to withstand high temperatures and resist wear makes it indispensable.
My first brush with aerospace-grade ceramics was when I had a friend working at an aerospace firm. He showed me how Al₂O₃-based thermal barriers were applied to turbine blades. Even a slight improvement in heat resistance can translate into better fuel efficiency and engine performance.
5.2 Automotive and Transportation
In the automotive world, aluminum oxide abrasives and ceramic inserts are used to machine engine blocks, brake rotors, and other critical parts. Al₂O₃ coatings also appear in certain wear-prone surfaces.
I had a chance to see how advanced brake pads sometimes incorporate Al₂O₃ particles for increased friction stability. This keeps performance high even under the extreme heat generated by heavy braking.
5.3 Electronics and Semiconductor Industries
Aluminum oxide formula stands out as a fantastic electrical insulator. It’s used in substrates for circuit boards, insulating layers in microchips, and even protective layers in some display technologies.
When I worked briefly on a project involving semiconductor wafer processing, I realized that Al₂O₃ was often the go-to material for wafer carriers and protective shields in high-temperature reactors. The purity and cleanliness requirements in semiconductor manufacturing make ultra-pure Al₂O₃ essential.
5.4 Medical and Dental Fields
Biocompatibility is another advantage of Al₂O₃, making it suitable for implants and prosthetic devices. Ceramic hip joints and dental crowns often include aluminum oxide or zirconia-toughened alumina for improved strength and compatibility with the human body.
I remember visiting a dental lab where they used an Al₂O₃-based ceramic for crown substructures. These crowns had a reputation for being highly durable while blending cosmetically with real teeth once the porcelain overlay was applied.
5.5 Household Items and Consumer Goods
You might be surprised how often the aluminum oxide formula pops up in everyday items. Ceramic knives, for example, can contain Al₂O₃ or zirconia. High-quality sandpapers for home renovation projects often rely on Al₂O₃ grains.
I personally use Al₂O₃ abrasive papers for woodworking projects. When I compare them to less sophisticated abrasives, the difference in cut speed and longevity is quite noticeable. Plus, Al₂O₃ sandpapers give a more consistent finish over the life of the paper.
5.6 Construction and Infrastructure
Aluminum oxide-based refractories line the inside of furnaces, kilns, and reactors in construction and heavy industry. High-alumina bricks can withstand the extreme thermal and mechanical stress of cement kilns and steel-making furnaces.
During a plant tour of a steel mill, I noticed walls of the blast furnace lined with high-alumina bricks. The guide explained that the “aluminum oxide formula” was what kept these bricks stable at the furnace’s operating temperatures, which exceed 1,500 °C.
5.7 Abrasive Blasting and Surface Preparation
Surface preparation is a massive industry. Al₂O₃ grit is shot against metal surfaces to remove rust, old paint, or scale before repainting or coating. Because of its hardness, it’s effective and can be reused multiple times, making it more cost-effective in some scenarios than expendable media like crushed glass.
I once assisted a friend with a classic car restoration. We used Al₂O₃ blasting media to strip the old paint off the body panels. The result was a clean, matte finish ready for primer. The performance was much better compared to using simple sand, and it minimized the risk of embedding contaminants in the metal.
5.8 Data Table: Key Real-World Applications
Below is a table summarizing typical uses of aluminum oxide across various industries, highlighting why “aluminum oxide formula” is so versatile:
| Industry | Major Application | Why Al₂O₃ Works Well | Example Product/Process | Notes |
|---|---|---|---|---|
| Aerospace | Thermal barriers, engine parts | High temperature stability, wear resistance | Turbine blade coatings | Increases fuel efficiency |
| Automotive | Machining, brake components, spark plugs | High hardness, temperature resilience | Ceramic inserts, abrasive discs | Improves manufacturing efficiency |
| Electronics | Insulators, substrates, protective layers | Electrical insulation, chemical stability | Circuit boards, wafer carriers | Vital for high-purity environments |
| Medical/Dental | Implants, crowns, prosthetics | Biocompatible, high strength | Ceramic hip joints, dental crowns | Long-lasting, corrosion-resistant |
| Consumer Goods | Knives, sandpapers, scratch-resistant coatings | Hardness, durability | Ceramic knives, Al₂O₃ sandpaper | Better performance, longer life |
| Construction | Refractories for furnaces, kilns | Extreme thermal and structural stability | High-alumina bricks, castables | Essential for high-temperature lines |
| Surface Prep | Abrasive blasting, finishing | Hardness, reusability | Blasting media, metal cleaning | More efficient than basic sand |
| Semiconductor | Wafer processing equipment, insulating layers | High purity, insulation | Protective reactor linings, substrates | Critical in microchip fabrication |
(Table 5.1: Summary of aluminum oxide applications across major industries.)
5.9 Future Trends and Innovations
- Nano-Structured Al₂O₃: Research is ongoing into creating nano-scale alumina particles that could yield even higher strength and toughness in composite materials.
- Additive Manufacturing: 3D printing of ceramic components is a burgeoning field. Someday, we may see widespread 3D-printed Al₂O₃ parts.
- Bio-Inspired Materials: There’s interest in combining Al₂O₃ with organic polymers to create composites that mimic the layered structure of seashells, aiming for both strength and toughness.
I once attended a material science conference where a researcher showcased a prototype of a 3D-printed Al₂O₃ lattice structure. It was incredibly lightweight yet able to handle significant compressive loads. This might be a hint of what’s to come in advanced manufacturing.
5.10 My Observations on Industry Adoption
In my experience, large-scale adoption of Al₂O₃ solutions depends on cost-to-performance ratios. High-purity grades or advanced shaping methods can get pricey. Still, many manufacturers find that the long-term benefits—like extended tool life or reduced downtime—justify the investment.
It’s also been fascinating to see how synergy with other materials (like zirconia or silicon carbide) can make Al₂O₃ even more compelling. Hybrid or composite approaches often solve the brittleness issue while retaining the hardness and temperature resistance inherent to the aluminum oxide formula.
5.11 Chapter 5 Summary
From aerospace to the humble sandpaper sheet in your garage, Al₂O₃’s reach is vast. The aluminum oxide formula is a perfect example of how a single compound can adapt to a multitude of requirements, simply by altering purity, structure, or form.
As we continue, we’ll explore personal insights, best practices, and marketing angles—especially valuable if you’re considering selling or promoting Al₂O₃-based products. It’s not just about science; it’s about effectively communicating why Al₂O₃ stands out.
Chapter 6: Personal Insights, Workshop Revelations, and On-Site Encounters
6.1 How I Got Started with Al₂O₃
My own journey with aluminum oxide formula started innocently enough. I was restoring an old motorcycle and needed to clean and polish various metal parts. A more experienced friend recommended aluminum oxide sandpaper. The difference it made over basic hardware store sandpaper was night and day. That initial hands-on experience sparked my interest in the underlying properties of Al₂O₃.
I began reading about how aluminum oxide outperforms silicon carbide for certain applications, especially on metals. Soon, I found myself testing different abrasive products just to see which ones were best suited for tasks like deburring steel or polishing cast aluminum parts.
6.2 Unexpected Lessons in Abrasive Selection
One day, I decided to refurbish the cylinder head from my bike. I tried a low-quality abrasive that claimed to be “oxide-based.” The results were mediocre, and the paper wore out quickly. Switching to a reputable brand that clearly stated “high-purity aluminum oxide” on the packaging made a huge difference in finish consistency.
This taught me the importance of paying attention to abrasive grade and purity. The simple phrase “aluminum oxide formula” on a label does not guarantee quality. Reputable manufacturers often specify grit size, bond type, and percentage of Al₂O₃ purity. That level of detail is what ensures consistent performance.
6.3 Field Trip to a Ceramic Production Plant
As my fascination grew, I arranged a visit to a local ceramic production plant that specialized in Al₂O₃ parts. Walking around the plant, I saw massive presses forming green ceramic shapes and sintering furnaces roaring at over 1,500 °C. Talking to the engineers, I learned that controlling temperature uniformity in the furnace was critical. Even slight temperature gradients could result in warping or incomplete sintering.
Holding a freshly sintered Al₂O₃ component, I was struck by its smoothness and hardness. It felt nearly as hard as a gemstone. They let me do a quick scratch test on a piece of glass, and the Al₂O₃ part easily etched the surface, leaving a neat line behind.
6.4 Real-World Testing in an Automotive Shop
Later, a small automotive shop owner reached out, curious about switching to Al₂O₃-based grinding wheels. We tested these wheels on cast iron brake rotors and hardened steel crankshafts. The results were eye-opening:
- The Al₂O₃ wheels cut faster and stayed sharper longer.
- Surface finish improved, which was visible under a simple 10x magnifying glass.
- Workers appreciated the reduced heat buildup, although it was still essential to use coolant.
These tests solidified my belief that “aluminum oxide formula” is more than just a chemistry concept. When used correctly, it translates into real, measurable benefits like smoother finishes, faster machining, and cost savings over time.
6.5 Engaging with Hobbyists and Makers
I also interact with a lot of home-based hobbyists and makers. Many of them discover Al₂O₃ when they pick up sandpaper for wood or metal projects. They might not realize the significance at first. But once they compare standard sandpaper to Al₂O₃-based products, they appreciate how quickly it removes material and how long it lasts.
One maker friend, who was into knife forging, found that a set of Al₂O₃ belts for his belt grinder allowed him to shape steel knives more efficiently. He told me the belts retained their cutting ability, even under the intense friction and heat of shaping hardened steel. That’s something I’ve witnessed myself while sharpening chisels or lathe tools.
6.6 The Surprise of Ceramics in Daily Life
During a house renovation, I decided to install ceramic tiles in the kitchen. I was intrigued to learn that many ceramic tiles contain Al₂O₃ additives to enhance scratch resistance. In day-to-day life, these tiles resist scuffs from chairs or dropped utensils. This is yet another subtle example of “aluminum oxide formula” in action, making everyday objects more durable.
6.7 Visits to Trade Shows and Exhibitions
I’ve attended several industrial trade shows featuring materials and machining technologies. Walking through rows of booths, I often see a recurring theme of aluminum oxide. Some stands showcase the latest Al₂O₃ grinding wheels. Others highlight advanced ceramic tools or thermal spray coatings.
It’s fascinating how many company representatives emphasize the significance of the “aluminum oxide formula.” They’ll talk about its ionic bonds and crystalline structure in user-friendly language, explaining how these features lead to better products.
6.8 Conversations with Material Scientists
I’ve spoken with material scientists who are deeply involved in research on Al₂O₃ composites. One explained how doping a small percentage of zirconia into Al₂O₃ can drastically improve fracture toughness. Another was working on nano-scale grain boundary engineering to produce incredibly dense and strong Al₂O₃ ceramics.
From these conversations, I learned that the future of Al₂O₃ is not static. Researchers continuously refine it. The aluminum oxide formula remains the same, but we can optimize microstructures and doping elements to push performance boundaries further.
6.9 Personal Reflections on Durability and Reliability
Aluminum oxide has become my go-to solution for tough finishing tasks. I like how it rarely lets me down, whether I’m sanding, grinding, or cutting. Over time, I’ve grown to appreciate that reliability translates into saved time and fewer frustrations.
I also feel a sense of admiration for the old scientists and engineers who pioneered industrial Al₂O₃. The innovation behind harnessing such a stable, robust ceramic is impressive. It’s a testament to how understanding a compound’s fundamental chemistry can unlock wide-ranging benefits.
6.10 Lessons Learned and Advice for Newcomers
If you’re new to “aluminum oxide formula” and want to explore its potential:
- Choose Reputable Brands: Not all Al₂O₃ is created equal. Look for product lines that specify purity and grit size.
- Match the Tool to the Task: Whether you’re using Al₂O₃ as an abrasive or a ceramic tool, ensure it’s designed for your material and operating conditions.
- Respect Safety: Always wear appropriate protective gear. Al₂O₃ dust isn’t toxic per se, but airborne particulates of any kind can be harmful.
- Experiment Gradually: Start with smaller projects, get comfortable with the performance characteristics, then ramp up to more demanding tasks.
6.11 Chapter 6 Summary
My personal encounters highlight the versatility and reliability of Al₂O₃ in real settings, from motorcycle restorations to visits at ceramic plants. Each experience reaffirms what the broader industry data shows: that “aluminum oxide formula” is a powerful, adaptable foundation for countless products.
Hearing different perspectives—from hobbyists to scientists—has broadened my appreciation. What started as a mere curiosity turned into an ongoing journey of discovery, one that I expect to continue as new applications of Al₂O₃ keep emerging.
Chapter 7: Marketing Al₂O₃ Products and Where to Buy
7.1 The Rising Demand for Aluminum Oxide
Over the past decade, the global market for Al₂O₃ has expanded rapidly, driven by demand in automotive, electronics, aerospace, and even healthcare. When people search for terms like “aluminum oxide formula,” they’re often looking for technical specifications, best practices, or simply where to purchase quality products.
From a marketing standpoint, highlighting the versatility of Al₂O₃ is crucial. Because it’s so widely used, you can target different verticals—like abrasives, ceramics, coatings, or even niche hobby markets. Each requires its own messaging strategy.
7.2 Branding and Product Differentiation
If you’re selling Al₂O₃-based products, it’s important to differentiate yourself in a crowded market. Manufacturers often focus on:
- Purity Levels: High-purity Al₂O₃ commands a premium price. Emphasize the benefits like lower contamination and longer tool life.
- Unique Additives: Some companies incorporate dopants or advanced bonding methods to tailor performance.
- Processing Quality: Stress your brand’s state-of-the-art sintering, fusion, or coating techniques.
I’ve seen brands effectively leverage product certifications or lab test results to establish trust. For example, if your Al₂O₃ grinding wheels meet certain ISO or ANSI standards, make that a headline.
7.3 Distribution Channels
Al₂O₃ products are sold through various channels:
- Industrial Supply Houses: Cater to machining, metalworking, and construction industries.
- Online Retailers: Platforms like Amazon, Grainger, or specialized e-commerce sites that focus on abrasives or ceramics.
- Direct Sales: Some manufacturers choose direct sales or partnerships with large industrial buyers.
- Local Distributors: Smaller shops often prefer dealing with local distributors who can provide hands-on demonstrations.
When I tried to source specialized white aluminum oxide abrasives, I found that going through a reputable industrial supply house gave me more consistent quality compared to random online listings.
7.4 Pricing Strategies
Pricing for Al₂O₃ can fluctuate based on raw material costs (bauxite), energy prices, and market demand. You might consider:
- Tiered Pricing: Offer different grades at different price points—brown, white, pink, etc.
- Bulk Discounts: Encourage larger orders by reducing unit costs for bigger quantities.
- Value-Added Services: Provide custom grit sizes or specialized forming for higher margins.
I’ve noticed that premium Al₂O₃ products often justify their higher price with performance data. If you can show how a more expensive product saves time and money in the long run, customers are more willing to invest.
7.5 Marketing Collateral and Messaging
A successful Al₂O₃ marketing campaign should highlight:
- Performance Metrics: Hardness rating, temperature stability, and grit longevity.
- Applications: Tailor messages to each sector—mechanical, electrical, medical, etc.
- User Experience: Testimonials or case studies can bolster credibility.
- Sustainability: If your product has a reduced environmental impact or is recyclable, mention it.
I’ve seen small companies stand out by uploading detailed how-to videos. A short clip showing how an Al₂O₃ grinding wheel shapes metal can be incredibly persuasive, especially for new customers.
7.6 Where to Buy: A Quick Guide
If you’re looking to purchase Al₂O₃ products yourself, here are some broad categories:
- Hardware Stores: Many carry basic Al₂O₃ sandpapers and wheels.
- Industrial Suppliers: Your best bet for specialized abrasives, ceramic parts, and coatings.
- Online Platforms: Amazon, eBay, specialty abrasives sites. Be sure to check reviews and specifications to confirm authenticity.
- Direct from Manufacturers: If you need large volumes or custom formulations, going straight to the source can be cost-effective.
In my experience, local industrial suppliers are gold mines of expertise. Sometimes you’ll find older machinists or ceramics experts behind the counter who can offer invaluable advice based on your specific application.
7.7 Building Trust with Customers
For any marketer or seller of Al₂O₃ products, trust is vital. Customers need assurance that your product meets the advertised specs and will consistently perform. Provide detailed datasheets, including:
- Chemical Composition: Percentage of Al₂O₃, major and minor impurities.
- Grain Size and Morphology: Especially important for abrasives.
- Bonding Method (if applicable): Vitrified, resin-bonded, metal-bonded, etc.
- Testing Certificates: ISO, ANSI, or other relevant certifications.
When I was selecting an Al₂O₃ grinding wheel for finishing stainless steel, the most reassuring factors were the manufacturer’s transparent datasheet and their willingness to answer technical questions.
7.8 Challenges in Marketing Advanced Ceramics
Al₂O₃ is a technical product. The biggest challenge is bridging the knowledge gap for buyers who don’t speak “materials science.” That’s why I believe in using everyday language to explain how the aluminum oxide formula leads to real benefits—like faster cutting speeds, longer tool life, or more corrosion resistance.
I’ve seen companies fail because they relied too heavily on jargon and data without explaining the “so what?” factor for end-users. If someone is searching for “aluminum oxide formula,” they likely want an accessible explanation, not just a wall of numbers.
7.9 My Take on Successful Al₂O₃ Marketing
As someone who’s both used and studied Al₂O₃, I think successful marketing involves these steps:
- Educate: Offer free resources—blogs, videos, eBooks—that demystify aluminum oxide formula.
- Demonstrate: Show the product in action. Real-world demos build confidence.
- Quantify: Present the data that engineers and buyers care about.
- Connect: Engage with your audience through newsletters, forums, and social media groups.
I’ve personally responded better to brands that gave me tangible insight into how their products would solve my problems, rather than just listing features.
7.10 Chapter 7 Summary
The market potential for Al₂O₃ is substantial, and effective marketing hinges on clarity, credibility, and genuine value. Whether you’re a small business or a large manufacturer, the best approach is to meet customer needs with compelling, fact-based stories and accessible explanations of how the aluminum oxide formula improves their work.
FAQ
Q1: What is the chemical formula of aluminum oxide?
A: The chemical formula of aluminum oxide is Al₂O₃—often referred to simply as the “aluminum oxide formula.”
Q2: Does aluminum oxide rust like iron?
A: No, aluminum oxide doesn’t rust. Rust typically refers to iron oxidation. Al₂O₃ forms a protective layer on aluminum metal, preventing further corrosion.
Q3: Is aluminum oxide safe to use at home?
A: Generally, yes. Al₂O₃ is non-toxic and chemically stable. However, wear proper protective gear like masks and goggles when sanding or grinding with aluminum oxide abrasives, because airborne dust can be harmful if inhaled.
Q4: Where can I buy aluminum oxide products?
A: You can find them at hardware stores (for basic abrasives), industrial suppliers (for specialized forms), and online retailers. If you need large volumes or custom grades, buying directly from manufacturers might be more cost-effective.
Q5: Are there different types of Al₂O₃?
A: Yes, multiple forms (phases) exist, such as alpha, gamma, and more. Additionally, various purity levels (e.g., brown, white, pink) are tailored to specific applications like abrasives, ceramics, or coatings.
Q6: Is aluminum oxide formula the same as sapphire?
A: Essentially, sapphire is crystalline aluminum oxide (Al₂O₃) with trace impurities that give it color. So the formula is the same, but the crystal quality and purity are much higher for gem-grade sapphire.
Q7: What is the melting point of Al₂O₃?
A: Pure aluminum oxide has a melting point around 2,072 °C, which is part of what makes it ideal for high-temperature applications like refractories and some aerospace components.
Q8: Can I use Al₂O₃ abrasives on soft materials?
A: Absolutely. Al₂O₃ abrasives can be used on a wide range of materials, from wood and plastic to ferrous and non-ferrous metals. Just ensure you choose the appropriate grit and bond type.
Q9: Do I need specialized equipment to machine Al₂O₃ ceramics?
A: Yes. Fully sintered Al₂O₃ ceramics are extremely hard and often require diamond or CBN tooling. If you’re shaping a part in its “green” (pre-sintered) state, standard tools might suffice.
Q10: How does Al₂O₃ compare to silicon carbide (SiC)?
A: Both are hard, but Al₂O₃ is generally tougher, while SiC is more brittle but can offer better thermal conductivity in some cases. The choice depends on your specific application requirements.
Closing Thoughts
Aluminum oxide formula (Al₂O₃) isn’t just another chemical notation. It represents a material that’s deeply woven into modern life, from the abrasive in your sandpaper to cutting-edge aerospace components. I’ve come to value its reliability, versatility, and sheer performance in various real-world scenarios.
Whether you’re a hobbyist, an engineer, or a marketer promoting Al₂O₃ products, I hope this guide clarifies why aluminum oxide formula holds such an esteemed place. With its stability, hardness, and adaptability, Al₂O₃ continues to shape industries and spark innovative research. I’m confident that understanding it in detail—chemically, mechanically, and practically—can open doors for better projects, products, and solutions in your own pursuits.
Thank you for joining me on this deep dive into the world of aluminum oxide. If you have lingering questions, refer back to the FAQ or explore the specific chapters above. The next time you see “Al₂O₃” on a product label or hear someone mention “aluminum oxide formula,” you’ll know exactly why it’s such a big deal—and maybe you’ll share a bit of that knowledge with others, too.
Other Articles You Might Enjoy
- Is Aluminum Magnetic? Your Machining Guide to Aluminum Basics
Introduction: Why Care About Aluminum’s Magnetism? I’ve always been curious about how materials like aluminum shape the stuff we use every day—car parts, phone cases, even airplane wings. One question…
- 6061 Aluminum in Machining: Your Guide to Precision and Performance
Introduction: The Perfect Pairing of 6061 Aluminum and CNC I’ve always been fascinated by how everyday objects—like car parts or phone casings—start as raw materials and end up as precision-engineered…
- The Ultimate Guide to Anodized Aluminum Machining: Properties, Processes, and Applications
Chapter 1: Introduction to Anodized Aluminum Anodized aluminum is not just aluminum with a colorful finish. It is aluminum that has been treated through an electrochemical process to form a…
- Aluminum Angle Uncovered: Tips for Machining, Industry Application, and Beyond
Introduction: Unique Advantages of Aluminum Angle I’ve always been fascinated by the lightweight properties of aluminum angle and the sheer range of possibilities it opens up for different industries. Aluminum…
- Billet Aluminum Unlocked: Expert Machining Techniques and Real-World Applications
Introduction: Why Billet Aluminum Matters in Machining I’ve worked with various metals and alloys, but billet aluminum has always stood out for its potential and versatility. Billet aluminum refers to…
- The Complete Guide to Polishing Aluminum: Challenges and Solutions for Machinists
Introduction – Polishing Aluminum and Why It Matters I remember the first time I tried polishing aluminum. I wanted a clean, glossy finish but ended up with swirl marks and…
- Brushed Aluminum Demystified: From Elegance to Engineering
I’ve spent a fair amount of time working with different metals, and Brushed Aluminum has always caught my eye. It’s a material that combines an appealing, decorative surface with properties…
- Why Custom Aluminum Heat Sinks Are Best Made with CNC Machining
In today's advanced electronics and mechanical systems, aluminum heat sinks play a crucial role in thermal management, helping to dissipate heat and maintain optimal operating temperatures for components. As systems…
- Aluminum for Milling: Ultimate Alloy Machining Guide
The global aluminum milling market is expected to be worth USD 242 billion by 2027, with a CAGR of 5.7% over the forecast period. The aluminum milling industry's revenue is growing…
- Overcoming Deformation Issues in CNC Machining of Aerospace Aluminum Parts
In the aerospace industry, large aluminum alloy thin-wall structures are prized for their lightweight and high-strength properties. However, machining these components presents a set of unique challenges, primarily due to…