Electroplating is like giving metal parts a shiny, protective armor. Imagine turning a dull, rusty piece of metal into a gleaming, robust part ready to withstand the test of time. This transformation is crucial in various industries, especially in CNC machining, where precision and durability are paramount,it need to be strengthened by surface finishes technology. Copper and nickel plating are two popular methods used to enhance the durability of CNC machining parts. Let’s dive into the fascinating world of electroplating and explore how these metals work their magic.
Detailed Explanation of Copper Plating Methods
1. Cyanide Copper Plating
Cyanide copper plating involves the use of a cyanide-based solution to deposit a layer of copper onto the substrate. This method is widely used due to its ability to produce a uniform, fine-grained, and adherent copper deposit.
Principle:
The main components of cyanide copper plating solutions are copper cyanide and free cyanide. The copper cyanide acts as the source of copper ions, while the free cyanide stabilizes the solution and prevents the precipitation of copper hydroxide.
Chemical Reactions:
The main reactions in the cyanide copper plating process involve the formation of copper cyanide complexes. Initially, copper cyanide reacts with sodium cyanide to form sodium copper cyanide complexes. These complexes can exist in different forms depending on the amount of cyanide present. For instance, copper cyanide can react with one molecule of sodium cyanide to form sodium copper cyanide. If two molecules of sodium cyanide are present, it forms a different complex. The solution contains various cyanide complexes of copper that contribute to the plating process.
Process:
- The substrate is first cleaned and pickled to remove any surface oxides and contaminants.
- The part is then immersed in the cyanide copper plating bath.
- Direct current is applied, causing the copper ions to migrate towards the cathode (the part being plated) and deposit as a thin layer of copper.
Advantages:
- Produces a highly adherent and smooth copper layer.
- Good for plating over zinc die-cast and other difficult substrates.
- Can be used as a base layer for further electroplating processes.
Disadvantages:
- Highly toxic due to the use of cyanide, requiring careful handling and disposal.
- Environmental and safety concerns.
Typical Bath Composition:
| Component | Concentration |
|---|---|
| Copper cyanide | 20-40 g/L |
| Sodium cyanide | 30-60 g/L |
| Sodium carbonate | 30-60 g/L |
| Sodium hydroxide | 5-10 g/L |
Operating Conditions:
| Parameter | Value |
|---|---|
| Temperature | 20-30°C |
| Cathode current density | 1-3 A/dm² |
| Anode current density | 0.5-2 A/dm² |
| Agitation | Mild |
2. Acidic Sulfate Copper Plating
Acidic sulfate copper plating is another method widely used for depositing copper. It involves the use of a copper sulfate and sulfuric acid solution.
Principle:
This method relies on the use of copper sulfate as the source of copper ions and sulfuric acid to improve the solution’s conductivity.
Chemical Reactions:
The primary reaction is:

At the cathode:

Process:
- The part is cleaned and pickled similarly to the cyanide process.
- Immersed in an acid copper bath.
- Direct current is applied, causing copper ions to deposit on the substrate.
Advantages:
- Non-toxic compared to cyanide baths.
- Produces bright and smooth deposits.
- High efficiency and faster deposition rates.
Disadvantages:
- Higher internal stress in the deposited layer.
- Requires more precise control of bath composition and operating conditions.
Typical Bath Composition:
| Component | Concentration |
|---|---|
| Copper sulfate (CuSO₄·5H₂O) | 200-250 g/L |
| Sulfuric acid (H₂SO₄) | 50-100 g/L |
| Chloride ions (Cl⁻) | 50-100 ppm |
Operating Conditions:
| Parameter | Value |
|---|---|
| Temperature | 20-30°C |
| Cathode current density | 2-5 A/dm² |
| Agitation | Moderate |
Detailed Explanation of Nickel Plating Methods
1. Watts Nickel Plating
Watts nickel plating is one of the most common methods for nickel plating due to its simplicity and versatility.
Principle:
Watts bath contains nickel sulfate, nickel chloride, and boric acid. Nickel sulfate provides the nickel ions, nickel chloride improves conductivity and helps with anode corrosion, while boric acid acts as a buffer to maintain pH stability.
Chemical Reactions:
At the cathode:

Process:
- The substrate is cleaned and activated.
- Immersed in the Watts nickel plating bath.
- Direct current is applied, and nickel ions are reduced and deposited on the part.
Advantages:
- Produces smooth, ductile, and adherent nickel layers.
- Versatile and can be used for both decorative and functional applications.
Disadvantages:
- Requires precise control of pH and temperature.
Typical Bath Composition:
| Component | Concentration |
|---|---|
| Nickel sulfate (NiSO₄·6H₂O) | 240-300 g/L |
| Nickel chloride (NiCl₂·6H₂O) | 30-60 g/L |
| Boric acid (H₃BO₃) | 30-40 g/L |
Operating Conditions:
| Parameter | Value |
|---|---|
| Temperature | 45-60°C |
| Cathode current density | 2-5 A/dm² |
| pH | 4.0-4.5 |
| Agitation | Moderate |
2. Nickel Sulfamate Plating
Nickel sulfamate plating is known for producing low-stress, high-ductility nickel deposits.
Principle:
Nickel sulfamate is the main source of nickel ions. It is preferred for applications requiring low internal stress and high ductility.
Chemical Reactions:
At the cathode:
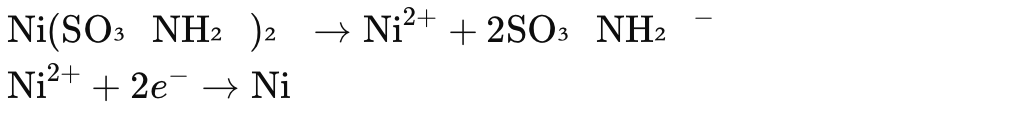
Process:
- The part is cleaned and pickled.
- Immersed in the nickel sulfamate plating bath.
- Direct current is applied, and nickel ions are deposited onto the substrate.
Advantages:
- Produces deposits with low internal stress.
- Suitable for thick deposits and applications requiring high ductility.
Disadvantages:
- More expensive than Watts bath.
- Requires precise control of bath composition and conditions.
Typical Bath Composition:
| Component | Concentration |
|---|---|
| Nickel sulfamate (Ni(SO₃NH₂)₂) | 300-450 g/L |
| Boric acid (H₃BO₃) | 30-40 g/L |
| Wetting agents | As required |
Operating Conditions:
| Parameter | Value |
|---|---|
| Temperature | 50-65°C |
| Cathode current density | 2-6 A/dm² |
| pH | 3.5-4.5 |
| Agitation | Moderate |
By understanding the detailed processes and conditions required for effective copper and nickel plating, manufacturers can enhance the durability, performance, and appearance of CNC machining parts, ensuring they meet the stringent demands of various industrial applications.
Other Articles You Might Enjoy
- High-Precision CNC Machining for Advanced Semiconductor Manufacturing
Introduction: CNC Machining and its Role in Advanced Semiconductor Manufacturing CNC machining, or Computer Numerical Control machining, refers to the process of subtracting material with high-speed precision machinery instructed by…
- Understanding and Exploring Bead Blasting in CNC Machining(copper plating Murray)
Bead blasting is an integral process in the realm of Computer Numerical Control (CNC) machining, often used for finishing or cleaning metal components. In simple terms, bead blasting involves leveraging…
- Mastering Chemical Coloring for CNC Machining Copper Parts
In the world of metal surface finishes, chemical coloring has gained significant traction, especially for aluminum and copper. This technique not only enhances the aesthetic appeal of the parts but…
- Requirements for CNC Machining Parts
Preparation Work Complete the necessary preparation before machining, including process analysis, process route design, tool and fixture selection, and program compilation. online cnc machining service Operating Steps and Contents Start…
- What are the requirements for CNC machining of bearing parts?
Bearings are common and important parts in the automotive industry, which can support transmission components and transmit torque. Generally, CNC machining centers are used to process bearing parts. So what…
- Evolution of Mills and Machining Centers: The Future of CNC Machining Parts
Stepping into the world of CNC machining, you quickly realize how pivotal mills and machining centers are in crafting precise parts. Over time, these machines have evolved significantly, transforming from…